Paroxysmal Nocturnal Hemoglobinuria Explained
Paroxysmal nocturnal hemoglobinuria affects blood cells and may lead to hemoglobinuria, fatigue, or shortness of breath. Since symptoms can be missed, learning the common causes and the standard diagnostic workup can help you prepare for a medical visit.

Paroxysmal nocturnal hemoglobinuria is a complex blood disorder that affects how red blood cells survive in the bloodstream. Although it is rare, it can have serious consequences, from persistent fatigue to life threatening blood clots and kidney problems. Understanding how and why it develops helps make sense of its symptoms and the medical tests and treatments that doctors use.
This article is for informational purposes only and should not be considered medical advice. Please consult a qualified healthcare professional for personalized guidance and treatment.
What is paroxysmal nocturnal hemoglobinuria
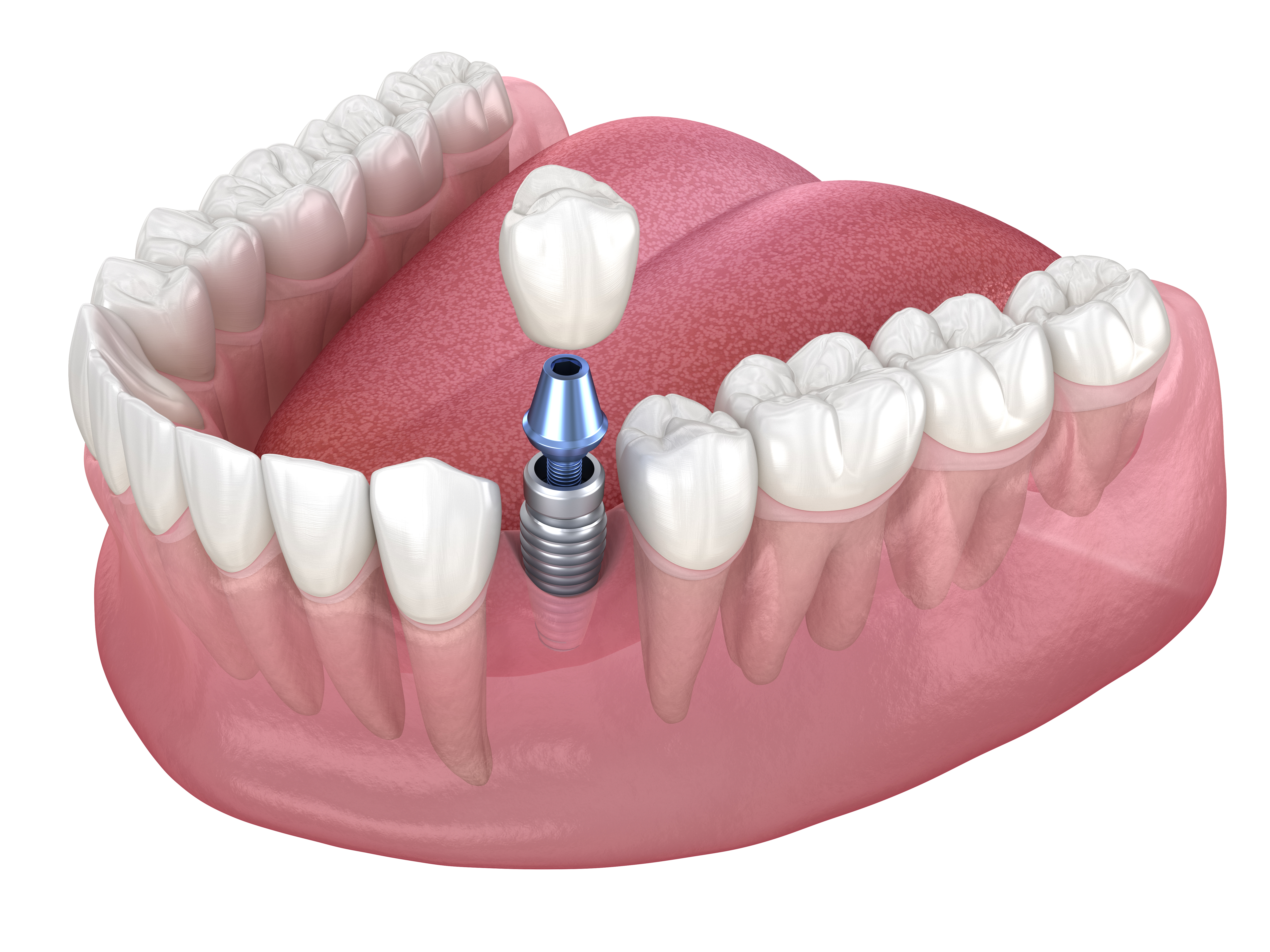
Paroxysmal nocturnal hemoglobinuria, or PNH, is an acquired disorder of blood forming stem cells in the bone marrow. It is not something a person is born with, and it is not contagious. A genetic mutation arises in a stem cell that gives rise to blood cells. As that mutated cell multiplies, it creates a population or clone of red blood cells, white blood cells, and platelets that lack certain protective proteins on their surface.
These missing proteins normally shield red blood cells from attack by a part of the immune system called the complement system. Without this protection, complement mistakenly targets the affected red blood cells and destroys them inside the bloodstream. This process is called intravascular hemolysis. When many red cells are broken down at once, a large amount of hemoglobin is released and cleared through the kidneys, which can lead to dark or reddish urine.
Over time, PNH can cause chronic anemia, a higher risk of blood clots, and sometimes low levels of other blood cells. It often occurs on its own but may also appear in people who have or had bone marrow failure syndromes such as aplastic anemia.
PNH and aHUS: how are they connected
The phrase PNH and aHUS refers to two distinct but related conditions that both involve abnormal activation of the complement system. Atypical hemolytic uremic syndrome, or aHUS, is usually driven by inherited or acquired abnormalities in complement regulation that mainly damage small blood vessels, especially in the kidneys.
In PNH, the core problem lies in the bone marrow stem cells that produce blood cells without key protective surface proteins. In aHUS, the problem usually involves complement control proteins in the blood or on vessel walls, leading to clotting and damage in tiny vessels. Both conditions may cause hemolysis and anemia, and both may lead to kidney problems, but their patterns and triggers differ.
Because of these shared complement pathways, some of the same medicines can be used to control complement activity in both PNH and aHUS. However, the way doctors diagnose and monitor these conditions is different, and treatment plans are tailored to the specific disorder and to the person living with it.
Recognizing hemoglobinuria symptoms in PNH
Hemoglobinuria symptoms are a key feature of PNH and give the disorder part of its name. Hemoglobinuria means that hemoglobin, the red pigment from inside red blood cells, appears in the urine. People with PNH may notice urine that looks dark, tea colored, cola colored, or reddish, especially in the morning after the urine has concentrated overnight.
Dark urine is only one aspect of PNH. Ongoing destruction of red blood cells can lead to significant anemia, which often causes fatigue, weakness, shortness of breath with mild exertion, pale skin, and rapid heartbeat. Some people experience headaches, chest pain, or difficulty concentrating because tissues are not getting enough oxygen.
PNH is also associated with an increased tendency to develop blood clots in unusual locations, such as abdominal veins or brain veins. Symptoms of clotting may include sudden abdominal pain, swelling in a limb, severe headaches, or neurologic changes. Other possible symptoms include abdominal pain not clearly linked to clots, difficulty swallowing, and in men, erectile dysfunction. The pattern and severity of symptoms can vary widely between individuals.
Hemoglobinuria causes in PNH and other conditions
Hemoglobinuria causes in PNH are directly tied to intravascular hemolysis. Because affected red blood cells lack protective surface proteins, complement punches holes in their membranes. Hemoglobin spills into the plasma and is filtered by the kidneys into the urine. Episodes of increased hemolysis may be triggered by infections, surgery, certain medications, or other physical stresses.
It is important to remember that hemoglobinuria is not unique to PNH. Other causes include severe transfusion reactions, certain infections, mechanical damage to red blood cells from medical devices such as heart valves, some inherited red cell disorders, and intense physical exertion in rare cases. Reddish or brown urine can also result from muscle breakdown products, foods, or drugs.
Because many different conditions can cause discolored urine, anyone who notices persistent dark or red urine should seek medical evaluation. Doctors use urine tests, blood tests, and a careful medical history to distinguish between hemoglobin, blood, and other pigments and to track down the underlying cause.
How PNH is diagnosed and monitored
Diagnosis of PNH usually starts when a person presents with unexplained anemia, dark urine, unusual blood clots, or signs of bone marrow failure such as low white cells or platelets. Basic blood tests often show anemia and signs of hemolysis, such as elevated lactate dehydrogenase, low haptoglobin, and increased bilirubin. Kidney function tests may show damage if hemoglobin has stressed the kidneys.
The key diagnostic test for PNH is flow cytometry on blood cells. This method looks for the absence of specific surface proteins that depend on a structure called a GPI anchor. Red blood cells and white blood cells lacking these GPI anchored proteins confirm the presence of a PNH clone, and the size of that clone helps doctors judge how extensive the disorder is.
Once PNH is diagnosed, regular monitoring is important. This typically includes repeated blood counts, markers of hemolysis, kidney and liver function tests, and assessment for any new symptoms suggesting clotting or organ damage. In some individuals, PNH coexists with or evolves into other bone marrow disorders, so follow up with a hematology specialist is usually long term.
Treatment and long term outlook for PNH
Although the headline focus is on explaining PNH, understanding its management gives additional context. Historically, treatment centered on supportive care, including blood transfusions for anemia, folic acid supplementation, iron management, and sometimes blood thinners for people at high risk of clots. These measures remain important for many patients.
In recent years, medicines that block the complement system have transformed the outlook for many people with PNH. By inhibiting specific complement components, these therapies reduce intravascular hemolysis, lower the risk of blood clots, and can improve fatigue and quality of life. Because they change how the immune system works, they require careful monitoring and vaccination against certain infections.
For a small number of people, a stem cell or bone marrow transplant may be considered, particularly when PNH occurs with severe bone marrow failure. Transplant is currently the only approach that can potentially eliminate the PNH clone, but it carries significant risks and is not suitable for everyone. Decisions about treatment depend on symptom severity, lab findings, other health conditions, and personal preferences.
With appropriate specialist care, many individuals with PNH can live for many years. Ongoing research continues to refine complement targeting therapies and explore new strategies. For anyone affected, close partnership with a hematologist, attention to hemoglobinuria symptoms, and regular monitoring are central to managing this rare but important blood disorder.